A clinical candidate being evaluated for aminoglycoside ototoxicity
You never know what you will find in the literature. A search for new antibacterials that have entered clinical trials identified ORC-13661, which I had not previously heard of. Despite being involved with antibacterial R&D for many years, I knew little about drugs being developed to help reduce hearing loss.
Background. Although aminoglycoside antibiotics have broad spectrum activity against most pathogens, their use is usually restricted due to potential side effects that include ototoxicity (irreversible hearing and balance problems) and nephrotoxicity (kidney damage). This is also ototoxicity issues with other drugs such as anticancer drug cisplatin. Therefore, therapies that could be co-administered with any potential ototoxic drugs could significantly reduce or even eliminate hearing loss. A review of hearing loss treatments currently in development and future perspectives was recently published for those interested.1
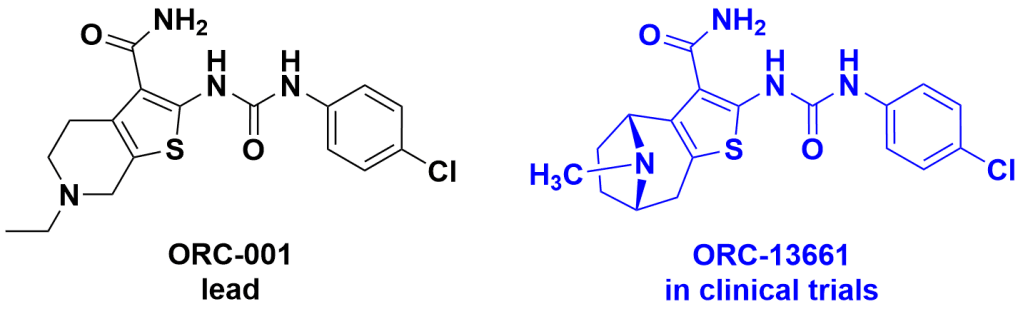
Discovery of ORC-13661 and proposed mechanism. Researchers at The University of Washington used a zebrafish neuromast hair cell protection assay to look for compounds that protect these mechanosensory hair cells in free-swimming larvae against aminoglycoside (neomycin)-induced cell death.2 These cells are very sensitive to aminoglycoside associated damage or death and can be used to identify both enhancers and suppressors of ototoxic activity. One of the small molecules identified in the zebrafish screen was the thiophene-urea carboxamide ORC-001. Although ORC-001 was active in an in vivo aminoglycoside-induced hearing loss rat model, improvements were required in solubility, oral bioavailability, and half-life, as well as reduced hERG inhibition. Additionally, ORC-001 exhibited gradual partial air mediated oxidation, which is not ideal for a potential drug. As part of this study, over 400 analogues, including amines and quaternary ammonium salts, were synthesised, and evaluated in the zebrafish assay with ORC-13661 chosen for clinical development (US9,493,482 patent). Further studies showed that ORC-13661 was able to protect sensory hair cells from aminoglycoside and cisplatin ototoxicity in in vitro and in vivo studies.3,4 The mechanism by which ORC-13661 reduces cell toxicity is based on direct competition with aminoglycosides for access to the mechanoelectrical transducer (MET) channel, and for cisplatin by a MET-dependent mechanism.3
Clinical development of ORC-13661. The University of Washington licensed ORC-13661 to Oricula Therapeutics (Seattle, WA, USA), who announced the successful completion of a phase-I ascending dose safety study in August 2018. Oricula licensed ORC-13661 to Decibel Therapeutics (Boston, MA, USA; development code DB-041) in September 2018, but no further clinical evaluation was undertaken. However, a new phase-II trial (NCT05730283) was recently registered by the Oregon Health and Science University, which is scheduled to start in mid-2024. This trial will evaluate whether hearing loss can be prevented in patients with non-tuberculosis Mycobacteria (NTM) infections undergoing treatment with intravenous (IV) amikacin (aminoglycoside). It will be interesting to follow this trial and others in the hearing loss area.
References.
- Joey Lye et al., Recent therapeutic progress and future perspectives for the treatment of hearing loss, Biomedicines, 2023, 11, 3347.
- Sarwat Chowdhury et al., Phenotypic optimization of urea−thiophene carboxamides to yield potent, well tolerated, and orally active protective agents against aminoglycoside-induced hearing loss, J. Med. Chem., 2018, 61, 84−97.
- Siân R. Kitcher, et al. ORC-13661 protects sensory hair cells from aminoglycoside and cisplatin ototoxicity. JCI Insight, 2019, 4, e126764.
- Joseph A. Bellairs et al., An in vivo biomarker to characterize ototoxic compounds and novel protective therapeutics, Front. Mol. Neurosci., 2022, 15, 944846.