As part of my work with the Institute for Molecular Bioscience at The University of Queensland, an update to our “Antibiotics in Clinical Trials” reviews that have been published in The Journal of Antibiotics every few years since 2011. The good news is that it’s ‘Open Access’ – click here.
Some key points
- There are 47 direct-acting antibacterials, 5 non-traditional small molecule antibacterials, and 10 β-lactam/β-lactamase inhibitor (BLI) combinations under clinical development as of December 2022 (Total = 62)
- At the start of the pipeline, there are now more than double the number of phase-I candidates (26) compared to 2015 (11), while funding initiatives have also helped to boost the number of phase-II (25) compounds since 2019 (18) (see Figure below).
- Encouragingly, 16/26 (62%) of the compounds in phase-I and 14/25 (56%) in phase-II contain new pharmacophores.
- Two new small molecule antibacterial drugs first approved between 2020 and 2022: levonadifloxacin and its prodrug in India in 2020 and the oxazolidinone contezolid acefosamil in China in 2021. Recently, the sulbactam-durlobactam BLI combination was approved in the USA.
- One ‘non-traditional’ antibacterial, Rebyota, was approved in the USA in 2022 and recently, another, Vowst, was approved in the USA – both for C. difficile.
- Despite the encouraging trends in the early stage of the pipeline, further support will be needed to increase the number of new antibacterial drugs launched onto the market.
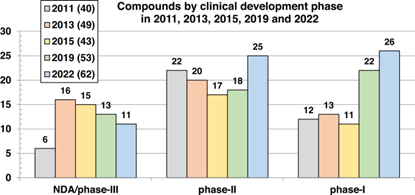
Figure. Comparison of the numbers of compounds undergoing clinical development as of 2011, 2013, 2015, 2019 and 2022 by development phase