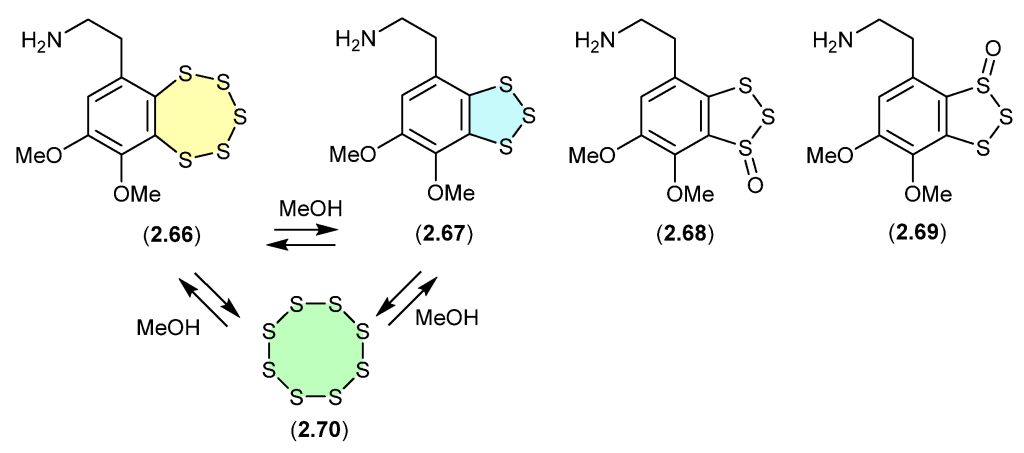
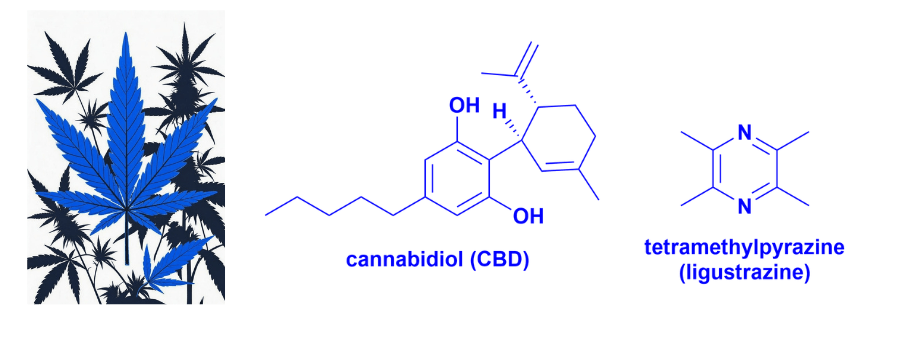
CBD’s path from a cannabis compound to a regulated pharmaceutical is a standout example of natural product drug development. In my recent Natural Product Reports review, CBD was noted as a component of Sativex® (THC/CBD extract). However, this undersells its potential, as CBD has also been approved as a standalone drug. This raises the question: “Where does pharmaceutical-grade CBD stand now?”
Approved CBD Medicine. Epidiolex® / Epidyolex® is highly purified plant-derived CBD dissolved in a sesame seed oil base, used to treat seizures in Dravet syndrome, Lennox–Gastaut syndrome,and Tuberous sclerosis complex. Marketed by Jazz Pharmaceuticals, it is approved for clinical use in countries such as the USA, Canada, UK, Switzerland, EU, Australia, and New Zealand.
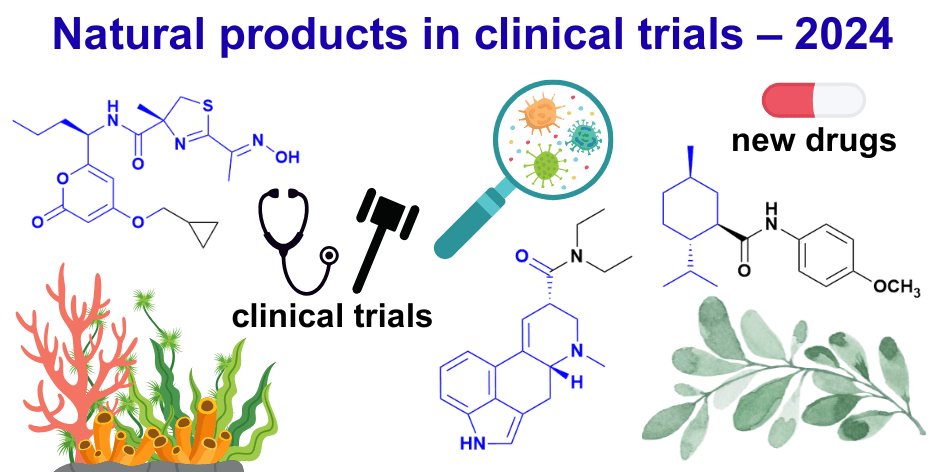
CBD in Clinical Trials. CBD remains active in early- to mid-stage studies (mostly Phase 1/2) across a variety of indications:
- CNS/psychiatric: anxiety (strongest data), autism, sleep
- Pain/inflammation: chronic pain, neuropathy
- Dermatology: acne, rosacea, atopic dermatitis
- Anti-infective: e.g., against MRSA
Innovative formulations highlight CBD’s potential, such as ART12.11 (Artelo’s CBD-tetramethylpyrazine cocrystal), which offers superior bioavailability and preclinical anxiety/depression benefits. Interestingly, tetramethylpyrazine (ligustrazine) is itself a bioactive plant natural product. Topical synthetic CBD candidates, such as BTX 1801 (and other formulations), have completed early clinical trials for anti-infective and anti-inflammatory activity, showing CBD’s versatility beyond CNS indications.
Conclusion. CBD transcends trends: it is clinically proven (Epidiolex), being investigated in diverse therapeutic areas, and is a key component of innovative formulations. Its story illustrates the regulatory and scientific challenges — and rewards — of natural product drug development. With the December 2025 U.S. Executive Order boosting medical marijuana and CBD research, accelerated progress can be expected.
#Cannabidiol #CBD #PharmaceuticalDevelopment #ClinicalTrials #NaturalProducts