Mark Butler and David Paterson
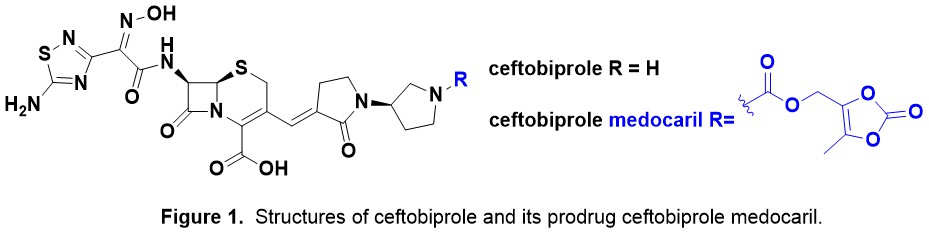
Background. Ceftobiprole medocaril (Zevtera®) has recently been in the news as Basilea Pharmaceutica was granted approval for Zevtera® by US FDA on April 2024 for treatment of adult patients with (1) methicillin-susceptible and methicillin-resistant Staphylococcus aureus (MSSA and MRSA) bacteraemia, including right-sided infective endocarditis, (2) acute bacterial skin and skin structure infections (ABSSSI) and (3) community acquired bacterial pneumonia (CABP). The structure of ceftobiprole (BAL 9141, Ro 63-9141) was first disclosed in a 1999 patent from Hoffmann La Roche and its prodrug ceftobiprole medocaril in 2001 (Fig. 1). Ceftobiprole has in vitro antimicrobial activity against a broad range of Gram-positive and Gram-negative pathogens. Notably this includes MRSA which is highly unusual for a cephalosporin. Ceftobiprole has a much higher affinity for PBP-2a than first, second, third and fourth generation cephalosporins.
Persistence Pays Off in the End. The development of Zevtera® has been a long time coming, with a number of clinical development roadblocks encountered along the way. Pivotal trials for complicated skin and skin structure infections (cSSSI), CABP, hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) were conducted at more than 150 sites from 2005 to 2007 in a collaboration between Basilea and J&J. Neither FDA nor EMA approval was granted at that time because of regulatory concerns related to clinical trial conduct at some trial sites. This led to a lawsuit against J&J, with a Dutch court eventually awarding Basilea $130 million for breach of the licence agreement. As a result, Basilea took total control of subsequent clinical trials – an ABSSSI trial was repeated and a landmark trial of ceftobiprole for complicated S. aureus bloodstream infections. It is these trials that led to the recent US FDA approval. Zevtera® has been previously approved in Canada and 14 European countries from 2013 with future expansion plans.
Medocaril/Medoxomil Antibiotic Prodrugs. Zevtera® is administered intravenously (IV) as the prodrug as ceftobiprole has low water solubility at physiological pH. Ceftobiprole medocaril is rapidly hydrolysed by plasma esterases and transported around the body. The related medoxomil prodrug moiety is present in lenampicillin and faropenem medoxomil (Fig. 2). This prodrug strategy is subtly different to using a prodrug to enhance oral dosing (e.g. contezolid acefosamil, Fig. 2).
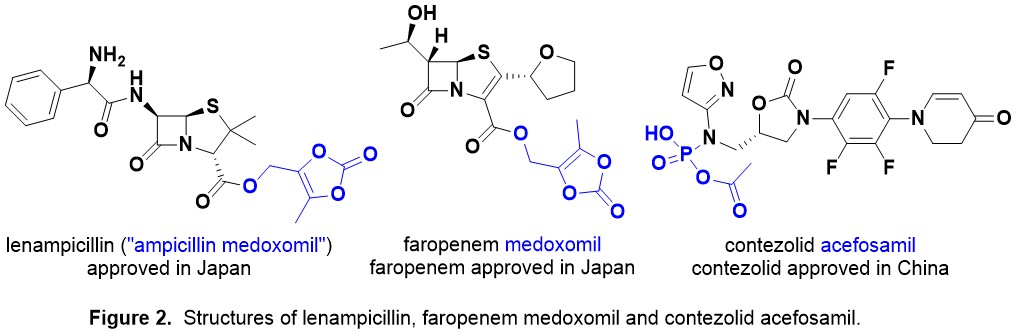
The carbamate containing medocaril and carbonate containing medoxomil prodrugs metabolise to give the drug, diacetyl (food flavour!) and carbon dioxide (CO2) – see a metabolism study for olmesartan medoxomil, which is an angiotensin II type 1 receptor antagonist used for antihypertension (Fig. 3). Bioactivation of this type of prodrug is due to esterase enzymes such as carboxylesterases, cholinesterases, and paraoxonases, which are widely distributed in biological fluids and tissues found throughout the body.